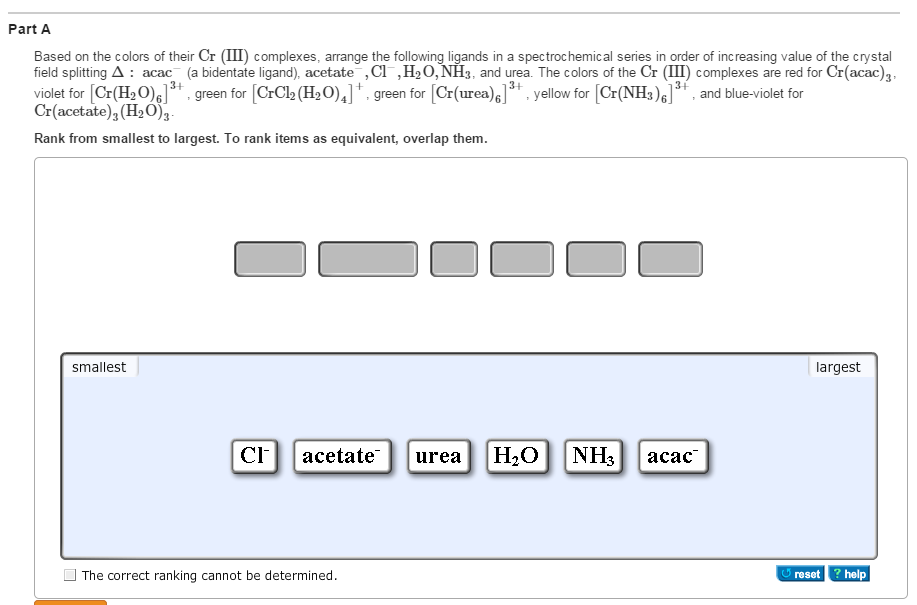
Rank Dmg In The Spectrochemical Series
A spectrochemical series is a list of ligands ordered on ligand strength and a list of metal ions based on oxidation number, group and its identity. In crystal field theory, ligands modify the difference in energy between the d orbitals (Δ) called the ligand-field splitting parameter for ligands or the crystal-field splitting parameter, which is mainly reflected in differences in color of similar metal-ligand complexes.
In this lab, you will synthesize a series of tetrahedral compounds using the same metal center (cobalt) and several of the ligands (Cl-, Br-, I-, and SCN-) from the Spectrochemical Series. These compounds will then be analyzed by UV-Vis spectroscopy to determine your own Spectrochemical Series (and whether it matches the one determined by others). Sep 06, 2019 Firstly, you should refer to the spectrochemical series. You may find that series in the chapter Complex Compounds. Now, generally the ligands at the beginning of the series are Weak Field Ligands and the ligands at the end are the Strong Field Ligands. But this is not the end.
Rank Dmg In The Spectrochemical Series Of 2016
Spectrochemical series of ligands[edit]
The spectrochemical series was first proposed in 1938 based on the results of absorption spectra of cobalt complexes.[1]
A partial spectrochemical series listing of ligands from small Δ to large Δ is given below. (For a table, see the ligand page.)
Your reputation is based on the quality and integrity of the products your organization manufactures and develops. It is essential to have the support of a strategic partner who understands that and provides you with the products you need. Experiment 1: Electronic Spectroscopy – Chromium(III) Complexes and the Spectrochemical Series Zehra Zaidi Date Submitted: 12th October 2011 Abstract A number of homoleptic chromium (III) complexes were synthesised, and their UV-vis spectra measured in order to construct a spectrochemical series for the ligands utilised (namely H 2 O, NH 3, acac-, Cl-and CN-) based on the relative magnitude.
I− < Br− < S2− < SCN− (S–bonded) < Cl− < N3− < F−< NCO− < OH− < C2O42− < O2−< H2O < acac− (acetylacetonate) < NCS− (N–bonded) < CH3CN < gly (glycine) < py (pyridine) < NH3 < en (ethylenediamine) < bipy (2,2'-bipyridine) < phen (1,10-phenanthroline) < NO2− < PPh3 < CN− < CO
Weak field ligand: H2O,F-,Cl-,OH-Strong field ligand: CO,CN-,NH3,PPh3
Ligands arranged on the left end of this spectrochemical series are generally regarded as weaker ligands and cannot cause forcible pairing of electrons within the 3d level, and thus form outer orbital octahedral complexes that are high spin. On the other hand, ligands lying at the right end are stronger ligands and form inner orbital octahedral complexes after forcible pairing of electrons within 3d level and hence are called low spin ligands.
Jan 22, 2016 What primary/secondary/melee would you suggest versus level 200 and above infested? I was going for tonkor with crit build and slash twin grakatas, but the damage seem to drop drastically after wave 60 in ODD:/ Builds on the weapons you suggest are appreciated too ^^. Best dmg vs high level infested.
However, keep in mind that 'the spectrochemical series is essentially backwards from what it should be for a reasonable prediction based on the assumptions of crystal field theory.'[2] This deviation from crystal field theory highlights the weakness of crystal field theory's assumption of purely ionic bonds between metal and ligand.
The order of the spectrochemical series can be derived from the understanding that ligands are frequently classified by their donor or acceptor abilities. Some, like NH3, are σ bond donors only, with no orbitals of appropriate symmetry for π bonding interactions. Bonding by these ligands to metals is relatively simple, using only the σ bonds to create relatively weak interactions. Another example of a σ bonding ligand would be ethylenediamine, however ethylenediamine has a stronger effect than ammonia, generating a larger ligand field split, Δ.
Ligands that have occupied p orbitals are potentially π donors. These types of ligands tend to donate these electrons to the metal along with the σ bonding electrons, exhibiting stronger metal-ligand interactions and an effective decrease of Δ. Most halide ligands as well as OH− are primary examples of π donor ligands.
When ligands have vacant π* and d orbitals of suitable energy, there is the possibility of pi backbonding, and the ligands may be π acceptors. This addition to the bonding scheme increases Δ. Ligands that do this very effectively include CN−, CO, and many others.[3]
Rank Dmg In The Spectrochemical Series Of Life
Spectrochemical series of metals[edit]
The metal ions can also be arranged in order of increasing Δ, and this order is largely independent of the identity of the ligand.[4]
Mn2+ < Ni2+ < Co2+ < Fe2+ < V2+ < Fe3+ < Cr3+ < V3+ < Co3+
In general, it is not possible to say whether a given ligand will exert a strong field or a weak field on a given metal ion. However, when we consider the metal ion, the following two useful trends are observed:
- Δ increases with increasing oxidation number, and
- Δ increases down a group.[4]
See also[edit]
References[edit]
- Zumdahl, Steven S. Chemical Principles Fifth Edition. Boston: Houghton Mifflin Company, 2005. Pages 550-551 and 957-964.
- D. F. Shriver and P. W. Atkins Inorganic Chemistry 3rd edition, Oxford University Press, 2001. Pages: 227-236.
- James E. Huheey, Ellen A. Keiter, and Richard L. Keiter Inorganic Chemistry: Principles of Structure and Reactivity 4th edition, HarperCollins College Publishers, 1993. Pages 405-408.
- ^R. Tsuchida (1938). 'Absorption Spectra of Co-ordination Compounds. I.'Bull. Chem. Soc. Jpn. 13 (5). doi:10.1246/bcsj.13.388.
- ^7th page of http://science.marshall.edu/castella/chm448/chap11.pdf
- ^Miessler, Gary; Tarr, Donald (2011). Inorganic Chemistry (4th ed.). Prentice Hall. pp. 395–396. ISBN978-0-13-612866-3.
- ^ abhttp://www.everyscience.com/Chemistry/Inorganic/Crystal_and_Ligand_Field_Theories/b.1013.php